 中文
中文



产品详情
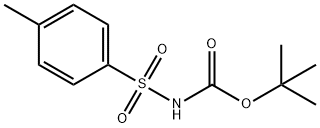
产品名称 英文名称:N-(tert-Butoxycarbonyl)-p-toluenesulfonamide 同义词 18303-04-3、N-(tert-Butoxycarbonyl)-p-toluenesulfonamide、tert-Butyl tosylcarbamate、N-Boc-p-toluenesulfonamide、tert-butyl N-(4-methylphenyl)sulfonylcarbamate、MFCD00134267、Carbamic acid, [(4-methylphenyl)sulfonyl]-, 1,1-dimethylethyl ester、N-Boc-4-tolylsulfo、N-Boc-对甲苯磺酰胺 产品性质 CAS编号:18303-04-3 分子式:C12H17NO4S 分子量:271.33 MDL号:MFCD00134267 PubChem编号:688170 别名:N-Boc-对甲苯磺酰胺 英文别名:18303-04-3|N-(tert-Butoxycarbonyl)-p-toluenesulfonamide|tert-Butyl tosylcarbamate|N-Boc-p-toluenesulfonamide|tert-butyl N-(4-methylphenyl)sulfonylcarbamate|MFCD00134267|Carbamic acid, [(4-methylphenyl)sulfonyl]-, 1,1-dimethylethyl ester|N-Boc-4-tolylsulfo 规格或纯度:>98.0% 英文名称:N-(tert-Butoxycarbonyl)-p-toluenesulfonamide 运输条件:常规运输 产品介绍:在 Mitsunobu 条件下可直接与伯醇、仲醇和烯丙醇发生偶联反应生成各种磺酰基保护胺。N-(tert-Butoxycarbonyl)-p-toluenesulfonamide may be used in the preparation of enyne amide, precursor for Pauson-Khand reaction.N-(tert-Butoxycarbonyl)-p-toluenesulfonamide is a N-substituted sulphonamide and its reaction with N-trityl L-serine esters under Mitsunobu reaction conditions is reported. It can be directly coupled with primary, secondary and allylic alcohols under Mitsunobu conditions to afford various sulfonyl-protected amines.N-(tert-Butoxycarbonyl)-p-toluenesulfonamide may be used in the preparation of enyne amide, precursor for Pauson-Khand reaction. PubChem SID:488191090 IUPAC Name:tert-butyl N-(4-methylphenyl)sulfonylcarbamate INCHI:InChI=1S/C12H17NO4S/c1-9-5-7-10(8-6-9)18(15,16)13-11(14)17-12(2,3)4/h5-8H,1-4H3,(H,13,14) InChi Key:DUTLOVSBVBGNDM-UHFFFAOYSA-N Canonical SMILES:CC1=CC=C(C=C1)S(=O)(=O)NC(=O)OC(C)(C)C Isomeric SMILES:CC1=CC=C(C=C1)S(=O)(=O)NC(=O)OC(C)(C)C WGK Germany:3 PubChem CID:688170 Reaxy-Rn:2858665 熔点:121-123°C 产品包装
免责声明:以上所展示的信息由企业自行提供,内容的真实性
、准确性和合法性由发布企业负责,chemdig对此不承担任何保证责任。 同时我们郑重提醒各位买/卖家,
交易前 请详细核实对方身份,切勿随意打款或发货,谨防上当受骗。如发现虚假信息,请向chemdig举报。 |
上海阿拉丁生化科技股份有限公司
|
上海阿拉丁生化科技股份有限公司
| 邮箱 | market@aladdin-e.com |
| 联系人 | 18521732826 |
咨询请告知是在ChemDig上看到的,有助于交易达成。

