 中文
中文



产品详情
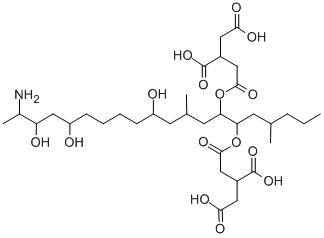
产品名称 英文名称:Fumonisin B1 同义词 fumonisin b1、116355-83-0、fumonisin-B1、Macrofusine、1,2,3-Propanetricarboxylic acid, 1,1'-[(1S,2R)-1-[(2S,4R,9R,11S,12S)-12-amino-4,9,11-trihydroxy-2-methyltridecyl]-2-[(1R)-1-methylpentyl]-1,2-ethanediyl] ester, (2R,2'R)-、3ZZM97XZ32、fumonisin B(1)、FB1、(R)-、烟曲霉毒素 B1 产品性质 CAS编号:116355-83-0 分子式:C34H59NO15 分子量:721.83 PubChem编号:2733487 别名:烟曲霉毒素 B1 英文别名:fumonisin b1|116355-83-0|fumonisin-B1|Macrofusine|1,2,3-Propanetricarboxylic acid, 1,1'-[(1S,2R)-1-[(2S,4R,9R,11S,12S)-12-amino-4,9,11-trihydroxy-2-methyltridecyl]-2-[(1R)-1-methylpentyl]-1,2-ethanediyl] ester, (2R,2'R)-|3ZZM97XZ32|fumonisin B(1)|FB1|(R)- 规格或纯度:≥98% (HPLC) 英文名称:Fumonisin B1 生化机理:真菌代谢物,可引起马脑白质软化症。霉菌毒素和蛋白丝氨酸/苏氨酸磷酸酶抑制剂(IC 50值为80μM(PP5),0.3(PP2Cα),0.4(PP2A),0.5(PP1γ2)和3 mM(PP2B))。细胞增殖抑制剂和凋亡诱导剂。 储存温度:-20°C储存,充氩 运输条件:超低温冰袋运输 备注:如果有可能,您尽量在使用的当天配置溶液,并在当天使用完它。但是,如果您需要预先配制储备溶液,我们建议您将溶液等份保存在-20°C的密封小瓶中。通常,它们最多可以使用一个月。在使用前和打开样品瓶之前,我们建议您让您的产品在室温下平衡至少1小时。需要更多关于溶解度,用法和处理的建议吗?请访问我们的常见问题(FAQ)页面以获取更多详细信息。 产品介绍:Fumonisin B1 is a fungal metabolite produced by Fusarium moniliforme, that has been shown to inhibit protein serine/threonine phosphatases (PP1, PP2A, PP2B, PP2C, and PP5/T/K/H), and is most effective with PP5. It has been shown to stimulate the activation of mitogen-activated protein kinase. Fumonisin B1 has been observed to inhibit sphingolipid biosynthesis, preferentially inhibiting sphingomyelin biosynthesis versus glycosphingolipids in neuronal cells. In primary rat liver cells, fumonisin B1 blocked biosynthesis of de novo sphingolipids throμgh LASS (ceramide synthase) inhibition. Caspase-3 activated or DNA fragmented apoptosis induced by fumonisin B1 binding to a TNF receptor has been documented in many human and rat cell lines. Fumonisin B1 has displayed immunotoxic effects by increasing expression of IL-1β, TNFα, IFN-γ; (interferon γ), IL-1α, IL-6, IL-10, IL-18 and IL-12 in varying mouse cells. Immunotoxicity of fumonisin B1 in human dendritic cells has been demonstrated throμgh expression of chemokine CXCL9 and IFN-γ. Further, fumonisin B1 is an activator of Akt.A serine/threonine phosphatase inhibitor.Fumonisin B1 is a fungal metabolite produced by Fusarium moniliforme, that has been shown to inhibit protein serine/threonine phosphatases (PP1, PP2A, PP2B, PP2C, and PP5/T/K/H), and is most effective with PP5. It has been shown to stimulate the activation of mitogen-activated protein kinase. Fumonisin B1 has been observed to inhibit sphingolipid biosynthesis, preferentially inhibiting sphingomyelin biosynthesis versus glycosphingolipids in neuronal cells. In primary rat liver cells, fumonisin B1 blocked biosynthesis of de novo sphingolipids through LASS (ceramide synthase) inhibition. Caspase-3 activated or DNA fragmented apoptosis induced by fumonisin B1 binding to a TNF receptor has been documented in many human and rat cell lines. Fumonisin B1 has displayed immunotoxic effects by increasing expression of IL-1β, TNFα, IFN-γ; (interferon γ), IL-1α, IL-6, IL-10, IL-18 and IL-12 in varying mouse cells. Immunotoxicity of fumonisin B1 in human dendritic cells has been demonstrated through expression of chemokine CXCL9 and IFN-γ. Further, fumonisin B1 is an activator of Akt.A serine/threonine phosphatase inhibitor. IUPAC Name:(2R)-2-[2-[(5R,6R,7S,9S,11R,16R,18S,19S)-19-amino-6-[(3R)-3,4-dicarboxybutanoyl]oxy-11,16,18-trihydroxy-5,9-dimethylicosan-7-yl]oxy-2-oxoethyl]butanedioic acid INCHI:InChI=1S/C34H59NO15/c1-5-6-9-20(3)32(50-31(44)17-23(34(47)48)15-29(41)42)27(49-30(43)16-22(33(45)46)14-28(39)40)13-19(2)12-24(36)10-7-8-11-25(37)18-26(38)21(4)35/h19-27,32,36-38H,5-18,35H2,1-4H3,(H,39,40)(H,41,42)(H,45,46)(H,47,48)/t19-,20+,21-,22+,23+,24+,25+,26-,27-,32+/m0/s1 InChi Key:UVBUBMSSQKOIBE-DSLOAKGESA-N Canonical SMILES:CCCCC(C)C(C(CC(C)CC(CCCCC(CC(C(C)N)O)O)O)OC(=O)CC(CC(=O)O)C(=O)O)OC(=O)CC(CC(=O)O)C(=O)O Isomeric SMILES:CCCC[C@@H](C)[C@H]([C@H](C[C@@H](C)C[C@@H](CCCC[C@H](C[C@@H]([C@H](C)N)O)O)O)OC(=O)C[C@@H](CC(=O)O)C(=O)O)OC(=O)C[C@@H](CC(=O)O)C(=O)O WGK Germany:3 RTECS:TZ8350000 PubChem CID:2733487 溶解性:Soluble in water (25 mg/mL), methanol (10 mg/mL), acetonitrile, and DMSO (10 mM). 敏感性:对光线和湿度敏感 象形图: 信号词:Warning 危险声明:H351 Suspected of causing cancer 预防措施声明:P280,P405,P501,P203,P318 个人防护装备:Eyeshields, full-face particle respirator type N100 (US), Gloves, respirator cartridge type N100 (US), type P1 (EN143) respirator filter, type P3 (EN 143) respirator cartridges 产品包装
免责声明:以上所展示的信息由企业自行提供,内容的真实性
、准确性和合法性由发布企业负责,chemdig对此不承担任何保证责任。 同时我们郑重提醒各位买/卖家,
交易前 请详细核实对方身份,切勿随意打款或发货,谨防上当受骗。如发现虚假信息,请向chemdig举报。 |
上海阿拉丁生化科技股份有限公司
|
上海阿拉丁生化科技股份有限公司
| 邮箱 | market@aladdin-e.com |
| 联系人 | 18521732826 |
咨询请告知是在ChemDig上看到的,有助于交易达成。


