 中文
中文



产品详情
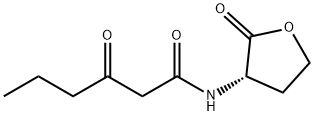
产品名称 英文名称:N-(Ketocaproyl)-L-homoserine Lactone 同义词 N-(3-氧代己酰)-L-高丝氨酸内酯、N-(β-酮己酰)-L-高丝氨酸内酯 产品性质 CAS编号:143537-62-6 分子式:C10H15NO4 分子量:213.23 MDL号:MFCD00171363 PubChem编号:688505 别名:N-(3-氧代己酰)-L-高丝氨酸内酯|N-(β-酮己酰)-L-高丝氨酸内酯 规格或纯度:≥98% 英文名称:N-(Ketocaproyl)-L-homoserine Lactone 应用:A component of quorum regulatory sensing. 储存温度:-20°C储存,充氩 运输条件:超低温冰袋运输 产品介绍:N-(β-ketocaproyl)-L-Homoserine lactone is a component of quorum regulatory sensing which is a regulatory system used by bacteria for controlling gene expression in response to increasing cell density. This regulatory process manifests itself with a variety of phenotypes including biofilm formation and virulence factor production. Coordinated gene expression is achieved by the production, release, and detection of small diffusible signal molecules called autoinducers. The N-acylated homoserine lactones (AHLs) comprise one such class of autoinducers, each of which generally consists of a fatty acid coupled with homoserine lactone (HSL). Regulation of bacterial quorum sensing signaling systems to inhibit pathogenesis represents a new approach to antimicrobial therapy in the treatment of infectious diseases. AHLs vary in acyl group length (C4-C18), in the substitution of C3 (hydrogen, hydroxyl, or oxo group), and in the presence or absence of one or more carbon-carbon double bonds in the fatty acid chain. These differences confer signal specificity throμgh the affinity of transcriptional regulators of the LuxR family. In one of the most-studied quorum-sensing systems in gram-negative bacteria, the LuxI AHL synthase catalyzes the production of N-(β-ketocaproyl)-L-homoserine lactone utilizing S-adenosylmethionine and hexanoyl-acyl carrier protein as reaction substrates in the marine bioluminescence bacterium V. fischeri. At increased populations of the bacteria, localized higher concentrations of 3-O-C6-HSL, an endogenous ligand to transcriptional factor LuxR, leads to increased production of both the AHL synthase and proteins responsible for bioluminescence. Numerous other species of bacteria also employ N-(β-ketocaproyl)-L-homoserine lactone in cell-to-cell communication.A component of quorum regulatory sensing.N-(β-ketocaproyl)-L-Homoserine lactone is a component of quorum regulatory sensing which is a regulatory system used by bacteria for controlling gene expression in response to increasing cell density. This regulatory process manifests itself with a variety of phenotypes including biofilm formation and virulence factor production. Coordinated gene expression is achieved by the production, release, and detection of small diffusible signal molecules called autoinducers. The N-acylated homoserine lactones (AHLs) comprise one such class of autoinducers, each of which generally consists of a fatty acid coupled with homoserine lactone (HSL). Regulation of bacterial quorum sensing signaling systems to inhibit pathogenesis represents a new approach to antimicrobial therapy in the treatment of infectious diseases. AHLs vary in acyl group length (C4-C18), in the substitution of C3 (hydrogen, hydroxyl, or oxo group), and in the presence or absence of one or more carbon-carbon double bonds in the fatty acid chain. These differences confer signal specificity through the affinity of transcriptional regulators of the LuxR family. In one of the most-studied quorum-sensing systems in gram-negative bacteria, the LuxI AHL synthase catalyzes the production of N-(β-ketocaproyl)-L-homoserine lactone utilizing S-adenosylmethionine and hexanoyl-acyl carrier protein as reaction substrates in the marine bioluminescence bacterium V. fischeri. At increased populations of the bacteria, localized higher concentrations of 3-O-C6-HSL, an endogenous ligand to transcriptional factor LuxR, leads to increased production of both the AHL synthase and proteins responsible for bioluminescence. Numerous other species of bacteria also employ N-(β-ketocaproyl)-L-homoserine lactone in cell-to-cell communication.A component of quorum regulatory sensing. IUPAC Name:3-oxo-N-[(3S)-2-oxooxolan-3-yl]hexanamide INCHI:InChI=1S/C10H15NO4/c1-2-3-7(12)6-9(13)11-8-4-5-15-10(8)14/h8H,2-6H2,1H3,(H,11,13)/t8-/m0/s1 InChi Key:YRYOXRMDHALAFL-QMMMGPOBSA-N Canonical SMILES:CCCC(=O)CC(=O)NC1CCOC1=O Isomeric SMILES:CCCC(=O)CC(=O)N[C@H]1CCOC1=O WGK Germany:3 PubChem CID:688505 溶解性:Soluble in ethanol, DMSO (~30 mg/ml), DMF (~30 mg/ml), methanol, and chloroform. 敏感性:对光及湿度敏感 个人防护装备:Eyeshields, Gloves, type N95 (US), type P1 (EN143) respirator filter 产品包装
免责声明:以上所展示的信息由企业自行提供,内容的真实性
、准确性和合法性由发布企业负责,chemdig对此不承担任何保证责任。 同时我们郑重提醒各位买/卖家,
交易前 请详细核实对方身份,切勿随意打款或发货,谨防上当受骗。如发现虚假信息,请向chemdig举报。 |
上海阿拉丁生化科技股份有限公司
|
上海阿拉丁生化科技股份有限公司
| 邮箱 | market@aladdin-e.com |
| 联系人 | 18521732826 |
咨询请告知是在ChemDig上看到的,有助于交易达成。

