 中文
中文



产品详情
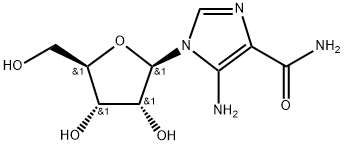
产品名称 英文名称:Acadesine 同义词 ACADESINE、2627-69-2、AICAR、AICA-riboside、Arasine、AICA riboside、AIC-Riboside、5-Amino-1-beta-D-ribofuranosyl-1H-imidazole-4-carboxamide、Acadesina、Acadesinum、AICAR (Acadesine)、GP-1-110、Protara、5-Amino-1-beta-D-ribofuranosylimidazole-4-carboxamide、5-Aminoimida、阿卡地新、5-氨基-1-beta-D-呋喃核糖基-1H-咪唑-4-甲酰胺、5-氨基咪唑-4-甲酰胺-1-B-D-呋喃核糖苷 产品性质 CAS编号:2627-69-2 分子式:C9H14N4O5 分子量:258.23 EC号:220-097-5 MDL号:MFCD00869751 PubChem编号:17513 别名:阿卡地新|5-氨基-1-beta-D-呋喃核糖基-1H-咪唑-4-甲酰胺|5-氨基咪唑-4-甲酰胺-1-B-D-呋喃核糖苷 英文别名:ACADESINE|2627-69-2|AICAR|AICA-riboside|Arasine|AICA riboside|AIC-Riboside|5-Amino-1-beta-D-ribofuranosyl-1H-imidazole-4-carboxamide|Acadesina|Acadesinum|AICAR (Acadesine)|GP-1-110|Protara|5-Amino-1-beta-D-ribofuranosylimidazole-4-carboxamide|5-Aminoimida 规格或纯度:≥98% 英文名称:Acadesine 生化机理:AICAR (AICA-Riboside) strongly inhibits the transcription of PPAR&alpha and the coactivation of PPAR&alpha. In adipocyte studies it has been shown to antagonize lipolysis induced by isoprenaline and has been sμggested for use in kinase cascade research. Additionally, research indicates that AICAR blocks the differentiation of 3T3-L1 (sc-2243) adipocytes. Studies demonstrate that AICAR can mimic the activity of insulin by activating AMPK (AMP-activated protein kinase), and affecting the expression of PEPCK-M (PEPCK) and glucose-6-phosphatase (G6Pase). The 5-aminoimidazole-4-carboxamide ribonucleoside (ZMP) is the monophosphorylated derivative of AICA-Riboside, and it can serve as the substrate for the aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). AICAR is an inhibitor of Hsp90, mTOR and p70 S6 Kinase.Cell-permeable activator of AMP-activated protein kinase. Is taken up into cells by adenosine transporters and phosphorylated by adenosine kinase to the active nucleotide ZMP (5-aminoimidazole-4-carboxamide ribonucleoside). 储存温度:-20°C储存 运输条件:超低温冰袋运输 备注:如果有可能,您尽量在使用的当天配置溶液,并在当天使用完它。但是,如果您需要预先配制储备溶液,我们建议您将溶液等份保存在-20°C的密封小瓶中。通常,它们最多可以使用一个月。在使用前和打开样品瓶之前,我们建议您让您的产品在室温下平衡至少1小时。需要更多关于溶解度,用法和处理的建议吗?请访问我们的常见问题(FAQ)页面以获取更多详细信息。 产品介绍:Acadesine导致ZMP的积累,Acadesine模仿AMP对AMPK和AMPK的刺激作用。An inhibitor of the transcription of PPARα, the coactivation of PPARα.Acadesine results in accumulation of ZMP, which mimics the stimulating effect of AMP on AMPK and AMPK kinase.An inhibitor of the transcription of PPARα, the coactivation of PPARα. ALogP:-2.2 PubChem SID:488182399 PubChem SID url:https//pubchem.ncbi.nlm.nih.gov/substance/488182399 IUPAC Name:5-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]imidazole-4-carboxamide INCHI:InChI=1S/C9H14N4O5/c10-7-4(8(11)17)12-2-13(7)9-6(16)5(15)3(1-14)18-9/h2-3,5-6,9,14-16H,1,10H2,(H2,11,17)/t3-,5-,6-,9-/m1/s1 InChi Key:RTRQQBHATOEIAF-UUOKFMHZSA-N Canonical SMILES:C1=NC(=C(N1C2C(C(C(O2)CO)O)O)N)C(=O)N Isomeric SMILES:C1=NC(=C(N1[C@H]2[C@@H]([C@@H]([C@H](O2)CO)O)O)N)C(=O)N WGK Germany:3 PubChem CID:17513 CAS Registry No.:2627-69-2 Wikipedia:Acadesine ChEMBL Ligand:CHEMBL1551724 DrugBank Ligand:DB04944 DrugCentral Ligand:37 溶解性:Soluble in water (9 mg/ml), and DMSO (75 mM). 敏感性:对湿度敏感 象形图: 信号词:Warning 危险声明:H315 Causes skin irritationH319 Causes serious eye irritationH335 May cause respiratory irritation 预防措施声明:P261,P305+P351+P338,P280,P302+P352,P321,P405,P501,P264,P271,P304+P340,P403+P233,P362+P364,P264+P265,P337+P317,P332+P317,P319 个人防护装备:dust mask type N95 (US), Eyeshields, Gloves 产品包装
免责声明:以上所展示的信息由企业自行提供,内容的真实性
、准确性和合法性由发布企业负责,chemdig对此不承担任何保证责任。 同时我们郑重提醒各位买/卖家,
交易前 请详细核实对方身份,切勿随意打款或发货,谨防上当受骗。如发现虚假信息,请向chemdig举报。 |
上海阿拉丁生化科技股份有限公司
|
上海阿拉丁生化科技股份有限公司
| 邮箱 | market@aladdin-e.com |
| 联系人 | 18521732826 |
咨询请告知是在ChemDig上看到的,有助于交易达成。


