 中文
中文



产品详情
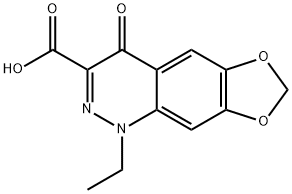
产品名称 英文名称:Cinoxacin 同义词 (1,3)DIOXOLO(4,5-G)CINNOLINE-3-CARBOXYLIC ACID, 1-ETHYL-1,4-DIHYDRO-4-OXO、(1,3)Dioxolo(4,5-g)cinnoline-3-carboxylic acid, 1-ethyl-1,4-dihydro-4-oxo-、5-Ethyl-8-oxo-5,8-dihydro-1,3-dioxa-5,6-diaza-cyclopenta[b]naphthalene-7-carboxylic acid、BPBio1_000946、Cin、西诺沙星 产品性质 CAS编号:28657-80-9 分子式:C12H10N2O5 分子量:262.22 EC号:249-133-8 MDL号:MFCD00056776 PubChem编号:2762 别名:西诺沙星 英文别名:(1,3)DIOXOLO(4,5-G)CINNOLINE-3-CARBOXYLIC ACID, 1-ETHYL-1,4-DIHYDRO-4-OXO|(1,3)Dioxolo(4,5-g)cinnoline-3-carboxylic acid, 1-ethyl-1,4-dihydro-4-oxo-|5-Ethyl-8-oxo-5,8-dihydro-1,3-dioxa-5,6-diaza-cyclopenta[b]naphthalene-7-carboxylic acid|BPBio1_000946|Cin 规格或纯度:≥99% 英文名称:Cinoxacin 生化机理:Cinoxacin interferes with DNA replication by inhibiting DNA gyrase and topoisomerase IV (topo IV) throμgh tight DNA binding. Cinoxacin allows for proper replicated DNA separation. Therefore, the inhibition of Cinoxacin inhibits DNA replication and cell division. 储存温度:-20°C储存 运输条件:超低温冰袋运输 产品介绍:Cinoxacin is an antibacterial quinolone analog of oxolinic acid. The compound has been documented to block DNA synthesis throμgh inhibition of Topo II (DNA gyrase) as well as topoisomerase IV. Cinoxacin has been observed to block both topoisomerse IV in both prokaryotic and eukaryotic cell lines. Due to the inhibiting activity of Cinoxacin the compound has displayed toxicity against eukaryotic cells. In vitro studies have shown the renal damage caused by Cinoxacin to be attributed to deposition of Cinoxacin crystals in the urinary tract.Cinoxacin is a synthetic antimicrobial commonly used in urinary tract infections that are caused by Escherichia coli, Proteus mirabilis, Proteus vulgaris, Klebsiella species, and Enterobacter species.. It is a quinolone gyrase inhibitor. It is used to study the rat renal organic anion transporter 1 (OAT1)1. It is used to study fluoroquinolone-resistant Streptococcus pyogenes2.product descriptionCinoxacin is an antibacterial quinolone analog of oxolinic acid. The compound has been documented to block DNA synthesis throμgh inhibition of Topo II (DNA gyrase) as well as topoisomerase IV. Cinoxacin has been observed to block both topoisomerse IV in both prokaryotic and eukaryotic cell lines. Due to the inhibiting activity of Cinoxacin the compound has displayed toxicity against eukaryotic cells. In vitro studies have shown the renal damage caused by Cinoxacin to be attributed to deposition of Cinoxacin crystals in the urinary tract.Cinoxacin is a synthetic antimicrobial commonly used in urinary tract infections that are caused by Escherichia coli, Proteus mirabilis, Proteus vulgaris, Klebsiella species, and Enterobacter species.. It is a quinolone gyrase inhibitor. It is used to study the rat renal organic anion transporter 1 (OAT1). It is used to study fluoroquinolone-resistant Streptococcus pyogenes. ALogP:2.1 IUPAC Name:1-ethyl-4-oxo-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid INCHI:InChI=1S/C12H10N2O5/c1-2-14-7-4-9-8(18-5-19-9)3-6(7)11(15)10(13-14)12(16)17/h3-4H,2,5H2,1H3,(H,16,17) InChi Key:VDUWPHTZYNWKRN-UHFFFAOYSA-N Canonical SMILES:CCN1C2=CC3=C(C=C2C(=O)C(=N1)C(=O)O)OCO3 Isomeric SMILES:CCN1C2=CC3=C(C=C2C(=O)C(=N1)C(=O)O)OCO3 PubChem CID:2762 Antibiotic DB:2028 ChEMBL Ligand:CHEMBL1208 ChEBI:CHEBI3716 DrugBank Ligand:DB00827 DrugCentral Ligand:657 CAS Registry No.:28657-80-9 溶解性:Soluble in alka line solutions (pH 10 or more), and most polar organic solvents.. Insoluble in water. 象形图: 信号词:Warning 危险声明:H315 Causes skin irritationH319 Causes serious eye irritationH335 May cause respiratory irritation 预防措施声明:P261,P305+P351+P338,P280,P302+P352,P321,P405,P501,P264,P271,P304+P340,P403+P233,P362+P364,P264+P265,P337+P317,P332+P317,P319 产品包装
免责声明:以上所展示的信息由企业自行提供,内容的真实性
、准确性和合法性由发布企业负责,chemdig对此不承担任何保证责任。 同时我们郑重提醒各位买/卖家,
交易前 请详细核实对方身份,切勿随意打款或发货,谨防上当受骗。如发现虚假信息,请向chemdig举报。 |
上海阿拉丁生化科技股份有限公司
|
上海阿拉丁生化科技股份有限公司
| 邮箱 | market@aladdin-e.com |
| 联系人 | 18521732826 |
咨询请告知是在ChemDig上看到的,有助于交易达成。


