中文
中文



产品详情
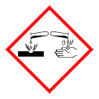
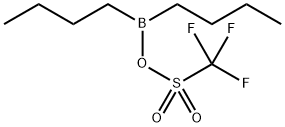
产品名称 英文名称:Dibutylboryl trifluoromethanesulfonate solution 同义词 60669-69-4、Dibutylboron trifluoromethanesulfonate、dibutylboranyl trifluoromethanesulfonate、DIBUTYLBORYL TRIFLUOROMETHANESULFONATE、Dibutylboron triflate、Dibutyl(((trifluoromethyl)sulfonyl)oxy)borane、Dibutylboron triflate; Dibutylboron trifluoromethanesulfo、二丁基硼三氟甲磺酸盐 溶液 产品性质 CAS编号:60669-69-4 分子式:C9H18BF3O3S 分子量:274.11 PubChem编号:2724243 别名:二丁基硼三氟甲磺酸盐 溶液 英文别名:60669-69-4|Dibutylboron trifluoromethanesulfonate|dibutylboranyl trifluoromethanesulfonate|DIBUTYLBORYL TRIFLUOROMETHANESULFONATE|Dibutylboron triflate|Dibutyl(((trifluoromethyl)sulfonyl)oxy)borane|Dibutylboron triflate; Dibutylboron trifluoromethanesulfo 规格或纯度:0.7mol/L in diethyl ether,Energyseal 英文名称:Dibutylboryl trifluoromethanesulfonate solution 储存温度:室温 运输条件:常规运输 备注:卖完停产,不再备货 产品介绍:Dibutylboryl trifluoromethanesulfonate (Dibutylboron triflate, Bu2BOTf) is an organometallic reagent. It efficiently promotes the1,4-addition of benzylic and allylic organocopper reagents.Dibutylboryl trifluoromethanesulfonate (Dibutylboron triflate, Bu2BOTf) may be used in the following studies ? Stereo- and regio-selective synthesis of erythro aldols. ? As promoter for the solution and polymer-supported trichloroacetimidate-based glycosylations. ? Synthesis of β-alkoxy carbonyl compounds, via one-step Mukaiyama aldol-type reaction. ? Aldol-type cyclization for the stereoselective synthesis of cyclic ethers. ? As reagent for the formation of boron enolates. ? As a complexation aid for the isolation of 1-acyldipyrromethanes.Dibutylboryl trifluoromethanesulfonate (Dibutylboron triflate, Bu2BOTf) is an organometallic reagent. It efficiently promotes the1,4-addition of benzylic and allylic organocopper reagents.Dibutylboryl trifluoromethanesulfonate (Dibutylboron triflate, Bu2BOTf) may be used in the following studies ? Stereo- and regio-selective synthesis of erythro aldols. ? As promoter for the solution and polymer-supported trichloroacetimidate-based glycosylations. ? Synthesis of β-alkoxy carbonyl compounds, via one-step Mukaiyama aldol-type reaction. ? Aldol-type cyclization for the stereoselective synthesis of cyclic ethers. ? As reagent for the formation of boron enolates. ? As a complexation aid for the isolation of 1-acyldipyrromethanes. IUPAC Name:dibutylboranyl trifluoromethanesulfonate INCHI:InChI=1S/C9H18BF3O3S/c1-3-5-7-10(8-6-4-2)16-17(14,15)9(11,12)13/h3-8H2,1-2H3 InChi Key:FAVAVMFXAKZTMV-UHFFFAOYSA-N Canonical SMILES:B(CCCC)(CCCC)OS(=O)(=O)C(F)(F)F Isomeric SMILES:B(CCCC)(CCCC)OS(=O)(=O)C(F)(F)F PubChem CID:2724243 象形图: 信号词:Danger 危险声明:H314 Causes severe skin burns and eye damageH318 Causes serious eye damage 预防措施声明:P280,P321,P405,P501,P264,P260,P301+P330+P331,P304+P340,P363,P264+P265,P305+P354+P338,P317,P302+P361+P354,P316 产品包装
免责声明:以上所展示的信息由企业自行提供,内容的真实性
、准确性和合法性由发布企业负责,chemdig对此不承担任何保证责任。 同时我们郑重提醒各位买/卖家,
交易前 请详细核实对方身份,切勿随意打款或发货,谨防上当受骗。如发现虚假信息,请向chemdig举报。 |
上海阿拉丁生化科技股份有限公司
|
上海阿拉丁生化科技股份有限公司
| 邮箱 | market@aladdin-e.com |
| 联系人 | 18521732826 |
咨询请告知是在ChemDig上看到的,有助于交易达成。