 中文
中文



产品详情
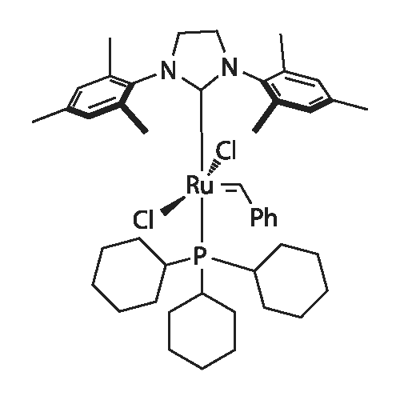
产品名称 英文名称:Grubbs Catalyst, 2nd Generation 同义词 246047-72-3、Grubbs Catalyst 2nd Generation、(1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium、Grubbs Catalyst, 2nd Generation、benzylidene-[1,3-bis(2,4,6-trimethylphenyl)imidazolidin-2-ylidene]-、Umicore Grubbs Catalyst M204、格拉布催化剂、Grubbs催化剂 产品性质 CAS编号:246047-72-3 分子式:C46H65Cl2N2PRu 分子量:848.97 MDL号:MFCD03453237 PubChem编号:11147261 别名:Umicore Grubbs Catalyst M204|格拉布催化剂|Grubbs催化剂 英文别名:246047-72-3|Grubbs Catalyst 2nd Generation|(1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium|Grubbs Catalyst, 2nd Generation|benzylidene-[1,3-bis(2,4,6-trimethylphenyl)imidazolidin-2-ylidene]- 规格或纯度:97% 英文名称:Grubbs Catalyst, 2nd Generation 储存温度:2-8°C储存,充氩 运输条件:冰袋运输 产品介绍:Grubbs第二代催化剂 和第一代催化剂相比更有活性,且具有更广泛的底物范围,包括对空间有要求或失活的烯烃,如1,1 - 二取代烯烃和α,β-不饱和羰基化合物ApplicationGrubbs Catalyst® M204 can be used as a catalyst for ring-closing metathesis (RCM), cross-metathesis, and ring-opening metathesis polymerization (ROMP). It is also used to synthesize trisubstituted olefins with excellent functional group tolerance and selectivity via cross-metathesis and ring closing metathesis reactions.It can also be used as a catalyst• To synthesize coumarins from phenolic compounds via RCM.• To cleave secondary (E)-allyl vic-diols to aldehydes.Grubbs 2nd Generation Catalyst Generally more active compared to 1st generation catalysts and with a broader substrate scope, including sterically demanding or deactivated olefins such as 1,1-disubstituted olefins and α,β-unsaturated carbonylsApplicationGrubbs Catalyst® M204 can be used as a catalyst for ring-closing metathesis (RCM), cross-metathesis, and ring-opening metathesis polymerization (ROMP). It is also used to synthesize trisubstituted olefins with excellent functional group tolerance and selectivity via cross-metathesis and ring closing metathesis reactions.It can also be used as a catalyst• To synthesize coumarins from phenolic compounds via RCM.• To cleave secondary (E)-allyl vic-diols to aldehydes.Product classM-P, Homogeneous Catalysts, N-Heterocyclic Carbene Ligands, M-C, Grubbs Catalyst® 2nd Generation, Carbene Ligands, SIMes, Phosphorus Ligands - AchiralReaction typeAlkene Metathesis, Acyclic Diene Metathesis, Cross Metathesis, Ring Arrangement Metathesis , Ring Closing Metathesis, Ring Opening Metathesis, Ring Opening Metathesis Polymerization, Self MetathesisChemical propertiesChemical formulaC46H65Cl2N2PRuEmpirical formula(SIMes)Ru(PCy3)(benzylidene)Cl2Molecular weight848.97MetalRuTheoretical metal content12Physical statepowderColororange-brownApplications & referencesAsymmetric synthesis of new cyclic fluorinated amino-acidsReference European Journal of Organic Chemistry, March 2018Photoinduced strain-assisted synthesis of a stiff-stilbene polymer by ring-opening metathesis polymerization.Reference Chem. Eur. J. 2020 (10.1002/chem.20200418)Facile synthetic route to [3.n]thiacyclophanes via ring-closing metathesis.Reference Eur. J. Org. Chem. 2020 (10.1002/ejoc.202000697)RCM-Based Total Synthesis of the Antibiotic Disciformycin B.Reference Angew. Chem. Int. Ed. 2020 (doi.org/10.1002/anie.202004589)Sequential two-fold Claisen Rearrangement, One-pot Ring Closing Metathesis and Cross-metathesis as a Route to Substituted Benzo[b]azepine-2-one, Benzo[b]azepine and Benzo[b]oxepine Derivatives.Reference Helv Chem Acta 2020 ASAP (doi.org/10.1002/hlca.202000216)Sequence-regulated vinyl polymers via iterative atom transfer radical additions and acyclic diene metathesis polymerization.Reference Polym Chem 2020 ASAP (doi.org/10.1039/D0PY01564D) IUPAC Name:benzylidene-[1,3-bis(2,4,6-trimethylphenyl)imidazolidin-2-ylidene]-dichlororuthenium;tricyclohexylphosphane INCHI:InChI=1S/C21H26N2.C18H33P.C7H6.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-7-5-3-2-4-6-7;;;/h9-12H,7-8H2,1-6H3;16-18H,1-15H2;1-6H;2*1H;/q;;;;;+2/p-2 InChi Key:FCDPQMAOJARMTG-UHFFFAOYSA-L Canonical SMILES:CC1=CC(=C(C(=C1)C)N2CCN(C2=[Ru](=CC3=CC=CC=C3)(Cl)Cl)C4=C(C=C(C=C4C)C)C)C.C1CCC(CC1)P(C2CCCCC2)C3CCCCC3 Isomeric SMILES:CC1=CC(=C(C(=C1)C)N2CCN(C2=[Ru](=CC3=CC=CC=C3)(Cl)Cl)C4=C(C=C(C=C4C)C)C)C.C1CCC(CC1)P(C2CCCCC2)C3CCCCC3 WGK Germany:3 PubChem CID:11147261 UN Number:1325 溶解性:Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Very Slightly, Heated 敏感性:对空气和光线敏感 熔点:143.5-148.5°C 象形图: 信号词:Warning 危险声明:H315 Causes skin irritationH319 Causes serious eye irritationH335 May cause respiratory irritationH228 Flammable solid 预防措施声明:P261,P305+P351+P338,P280,P370+P378,P210,P302+P352,P321,P405,P501,P240,P264,P271,P241,P304+P340,P403+P233,P362+P364,P264+P265,P337+P317,P332+P317,P319 个人防护装备:Eyeshields, Gloves, type N95 (US), type P1 (EN143) respirator filter 产品包装
免责声明:以上所展示的信息由企业自行提供,内容的真实性
、准确性和合法性由发布企业负责,chemdig对此不承担任何保证责任。 同时我们郑重提醒各位买/卖家,
交易前 请详细核实对方身份,切勿随意打款或发货,谨防上当受骗。如发现虚假信息,请向chemdig举报。 |
上海阿拉丁生化科技股份有限公司
|
上海阿拉丁生化科技股份有限公司
| 邮箱 | market@aladdin-e.com |
| 联系人 | 18521732826 |
咨询请告知是在ChemDig上看到的,有助于交易达成。



