 中文
中文



产品详情
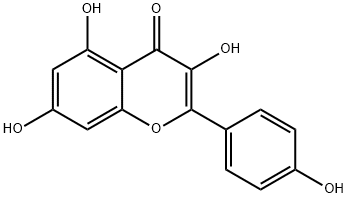
产品名称 英文名称:Kaempferol 同义词 5,7,4'-Trihydroxyflavonol、DTXCID30768、Indigo Yellow、KAEMPFEROL [INCI]、Kampferol、SPBio_003058、NSC656277、NSC-656277、3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one、Kaempherol、NCGC00179275-02、Kempferol、NCGC00016480-04、5,4'-Trihydroxyflavonol、MLS0010748、莰菲醇 产品性质 CAS编号:520-18-3 分子式:C15H10O6 分子量:286.24 Beilstein号:304401 MDL号:MFCD00016938 PubChem编号:5280863 别名:莰菲醇 英文别名:5,7,4'-Trihydroxyflavonol|DTXCID30768|Indigo Yellow|KAEMPFEROL [INCI]|Kampferol|SPBio_003058|NSC656277|NSC-656277|3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one|Kaempherol|NCGC00179275-02|Kempferol|NCGC00016480-04|5,4'-Trihydroxyflavonol|MLS0010748 规格或纯度:97% 英文名称:Kaempferol 生化机理:Description IC50 value Kaempferol inhibits proliferation of ovarian cancer cells at 40μM or higher concentrations[2]. Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is a relatively common nontoxic, natural dietary compound which has been reported to reduce the risk of ovarian cancer[1]. in vitro Kaempferol was found to inhibit estrogen receptor alpha expression in breast cancer cells and to induce apoptosis in glioblastoma cells and lung cancer cells by activation of MEK-MAPK. Studies have shown that kaempferol also has anti-inflammatory effects via inhibition of interleukin-4 and cyclo-oxygenase 2 expression by suppressing Src kinase and downregulating the NFκB pathway. Kaempferol is also effective in inhibiting angiogenesis and inducing apoptosis in ovarian cancer cells [1]. kaempferol has a distinct epigenetic activity by inhibition of histone deacetylases (HDACs). In silico docking analysis revealed that it fits into the binding pocket of HDAC2, 4, 7 or 8 and thereby binds to the zinc ion of the catalytic center. Further in vitro profiling of all conserved human HDACs of class I, II and IV showed that kaempferol inhibited all tested HDACs [4]. in vivo Male BALB/c mice with ALI, induced by intranasal instillation of LPS, were treated or not with Kae (100 mg/kg, intragastrically) 1h prior to LPS exposure. Kae treatment attenuated pulmonary edema of mice with ALI after LPS challenge, as it markedly decreased the lung W/D ratio of lung samples, protein concentration and the amounts of inflammatory cells in BALF[3]. Clinical trialN/A.Cell-permeable phytoestrogen and dietary flavonoid. Proapoptotic and protects against amyloid beta peptide neurotoxicity in vivo . Has many biological actions including inhibition of topoisomerase-1, MAO COX, estrogenic effects and activation of the mito 应用:一种黄酮醇。能使佛波醇酯处理的小鼠成纤维细胞或v-H-ras-转化的 NIH 3T3细胞变形后的恢复。能显著诱导核DNA退化,同时伴随脂类的过氧化反应。抑制拓扑异构酶I催化的DNA再连接。 储存温度:-20°C储存 运输条件:超低温冰袋运输 备注:如果有可能,您尽量在使用的当天配置溶液,并在当天使用完它。但是,如果您需要预先配制储备溶液,我们建议您将溶液等份保存在-20°C的密封小瓶中。通常,它们最多可以使用一个月。在使用前和打开样品瓶之前,我们建议您让您的产品在室温下平衡至少1小时。需要更多关于溶解度,用法和处理的建议吗?请访问我们的常见问题(FAQ)页面以获取更多详细信息。 产品介绍:一种黄酮醇。能使佛波醇酯处理的小鼠成纤维细胞或v-H-ras-转化的 NIH 3T3细胞变形后的恢复。能显著诱导核DNA退化,同时伴随脂类的过氧化反应。抑制拓扑异构酶I催化的DNA再连接。 IUPAC Name:3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one INCHI:InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H InChi Key:IYRMWMYZSQPJKC-UHFFFAOYSA-N Canonical SMILES:C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O Isomeric SMILES:C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O RTECS:LK9275200 PubChem CID:5280863 Reaxy-Rn:304401 溶解性:微溶于水;可溶于乙醚、乙醇 密度:1.688 敏感性:对光敏感、对空气敏感 熔点:276°C 象形图: 信号词:Danger 危险声明:H301 Toxic if swallowedH341 Suspected of causing genetic defects 预防措施声明:P321,P405,P501,P264,P281,P270,P330,P203,P301+P316,P318 Merck Index:5274 个人防护装备:Eyeshields,Faceshields,Gloves,type P2 (EN 143) respirator cartridges 产品包装
免责声明:以上所展示的信息由企业自行提供,内容的真实性
、准确性和合法性由发布企业负责,chemdig对此不承担任何保证责任。 同时我们郑重提醒各位买/卖家,
交易前 请详细核实对方身份,切勿随意打款或发货,谨防上当受骗。如发现虚假信息,请向chemdig举报。 |
上海阿拉丁生化科技股份有限公司
|
上海阿拉丁生化科技股份有限公司
| 邮箱 | market@aladdin-e.com |
| 联系人 | 18521732826 |
咨询请告知是在ChemDig上看到的,有助于交易达成。



